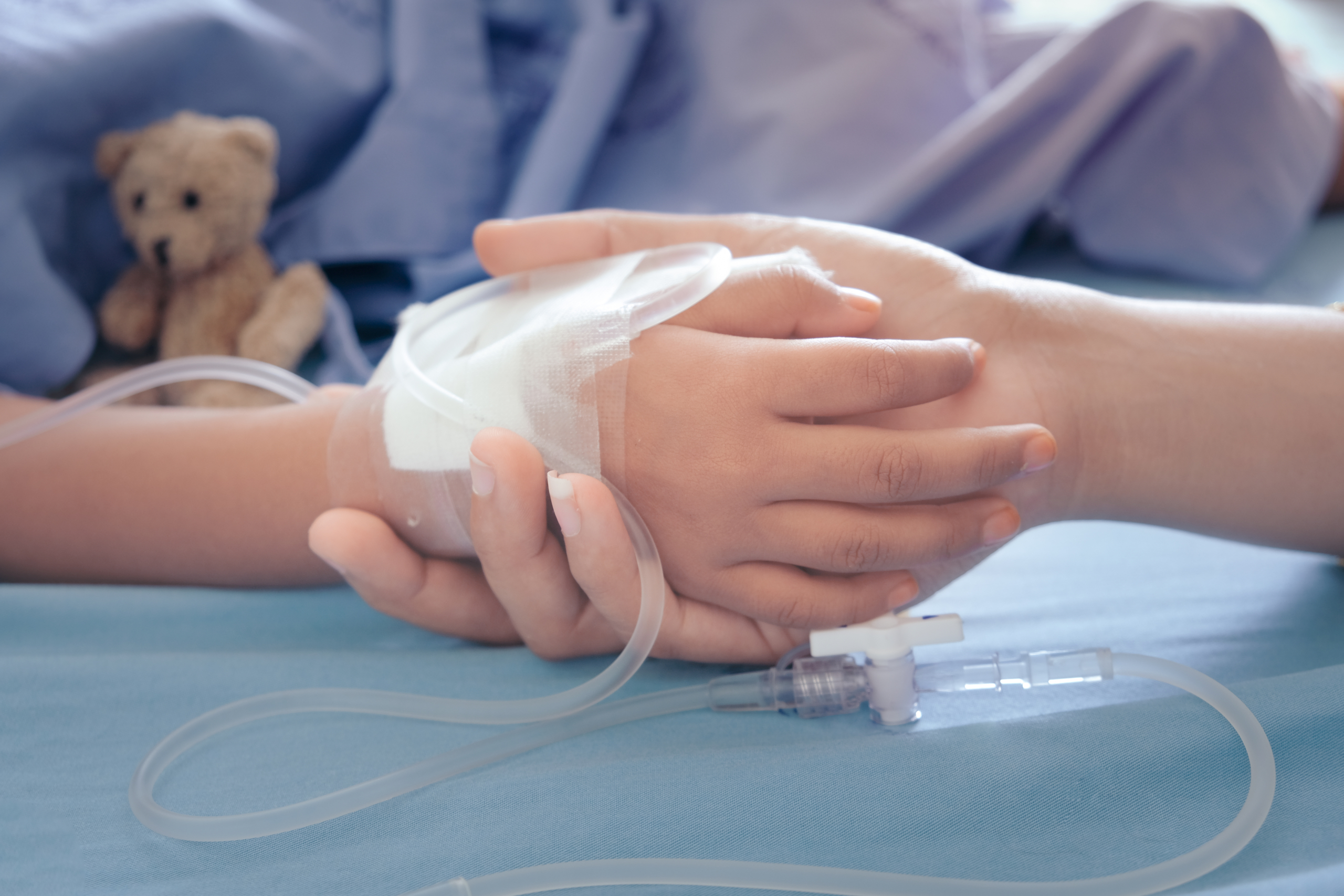
- Over 50% of children received unlicensed or off label medications in a hospital setting
- A renewed focus on clinical trials for children is urgently needed
- In4kids is Ireland’s National Children’s Clinical Trial Network; funded by the Health Research Board (HRB)
- In4kids is hosting a General Assembly Meeting on 6th June to discuss “Shaping the Future of Children’s Clinical Trials in Ireland”
Despite strong European regulations, a global meta-analysis has revealed that over half (56%) of hospitalised children receive unlicensed or off-label medications – including 49% in Europe. These stark findings highlight a systemic gap in safe, regulatory approved, tailored treatments for children.
To close this gap, In4kids – the HRB’s Irish Network for Children’s Clinical Trials is calling for a coordinated national effort to strengthen children’s clinical trial capacity across Ireland to ensure children have the same high-quality, evidence-based medicines and healthcare as adults.
The network, hosted at the INFANT Research Centre at University College Cork (UCC) connects hospitals, universities and clinical research centres to ensure children have access to innovative, evidence-based treatments.
“In4kids is developing the essential infrastructure to ensure that all children in Ireland benefit from world-class research and innovation—regardless of where they live. We are committed to ensuring that children and their families are active partners in shaping this journey”. – says Professor Geraldine Boylan, Lead In4kids, Professor of Neonatal Physiology, Director INFANT Research Centre at UCC
Why Children’s Clinical Trials Matter
Children’s bodies and minds develop differently to adults, requiring treatments that are researched, trialled, and regulatory approved just for them. In4kids supports this mission by helping researchers in Ireland conduct high-quality trials that meet ethical, regulatory and data protection standards.
As the Irish National Hub of conect4children -Stichting (c4c-S) – a large collaborative European children clinical trial network – In4kids is also attracting international studies to Ireland, ensuring that children in Ireland benefit from cutting-edge, international research and innovation.
Empowering Young People Through Research
In partnership with Children’s Health Ireland (CHI), In4kids established Ireland’s first National Young Person Advisory Group (YPAG) – a platform where young people contribute directly to research by shaping clinical trial design, reviewing materials, and advising on how results are communicated.
“This is research driven by children, for children,” says Professor Eleanor Molloy, Co-Lead In4kids, Consultant Neonatologist & Paediatrician, Professor and Chair of Paediatrics, Trinity College Dublin.
“The YPAG empowers young voices to be active research partners, not just participants. It’s a meaningful example of public and patient involvement which is now recognised as an integral part of research when research is conducted ‘with’ or ‘by’ children not ‘to’ or ‘about’ them”, she added.
Working Together for Better Outcomes
Building a strong and long-lasting children’s clinical trial network in Ireland requires collaboration – not just among researchers and clinicians but also with parents and young patients themselves.
By working together, it ensures that children’s voices are heard, evidence-based research is implemented, and families directly benefit from the latest treatments.
Clinical trials aren’t just for adults, they are for children too. Through this network, In4kids are ensuring that children’s needs shape clinical research, their voices influence change and Ireland leads the way in delivering safer, more effective treatment for the next generation.
If you’re interested in learning more about children’s clinical research in Ireland, In4kids are hosting a General Assembly Meeting on Friday 6th June in Trinity College Dublin from 10.00-16.00 to discuss “Shaping the Future of Children’s Clinical Trials in Ireland.” This event will bring together clinicians, researchers, policymakers, industry, and supporters to elevate the voice of children’s research in Ireland. Those interested in attending can register online on the In4kids website: https://in4kids.ie/general-assembly/
ENDS.
NOTES to Editor;
Reference to study:
About In4kids
In4kids is the Irish Network for Children’s Clinical Trials funded by the Health Research Board, hosted at INFANT Research Centre at University College Cork.
In4kids is the Irish Hub in the conect4children-Stichting (c4c-S) network which is a large collaborative European network that aims to facilitate the development of new drugs and other therapies for the entire paediatric population.